Last updated February 12, 2022
Please note: This page is no longer updated. Please check with your local government about which COVID-19 vaccines are available near you. If you live in the US, you can sign up for a COVID-19 vaccine, including boosters, at www.vaccines.gov.
Learn about how different COVID-19 vaccines work in our comprehensive guide.
Tweet
To visit the Spanish language version of this website, click here.
This page is a collaboration between Fancy Comma, LLC and Nidhi Parekh of The Shared Microscope. Keep reading to learn more about the COVID-19 vaccines currently in development.
Many different types of COVID-19 vaccines are being developed, and several have received FDA approval.
Here’s everything you need to know about the most promising COVID-19 vaccines, what they’re made of, and how they work. As these vaccines are proven safe and effective and obtain FDA approval (via Emergency Use Authorization or EUA), we’ll add that here, too.
Bookmark this page and check back often for updates.
Table of Contents:
- mRNA Vaccines for COVID-19
- Moderna
- Approved via Emergency Use Authorization by United States FDA for use in adults on Dec. 18, 2020
- Booster shots FDA approved via Emergency Use Authorization for all adults in the United States on Nov. 19, 2021
- Became the second COVID-19 vaccine to receive full FDA approval for use in people aged 18 or older on January 31, 2022. With full FDA approval, the Moderna COVID-19 vaccine can be marketed directly to US consumers. It will be sold under the trade name “Spikevax.”
- Pfizer/BioNTech
- Approved via Emergency Use Authorization by United States FDA for use in adults on Dec. 11, 2020
- Approved via Emergency Use Authorization by US FDA for use in adolescents (age 12-15) on May 10, 2021
- Became the first COVID-19 vaccine to receive full FDA approval for use in people aged 16 or older on August 23, 2021. With full FDA approval, the Pfizer/BioNTech vaccine can be marketed directly to US consumers. It will be sold under the trade name “Comirnaty.”
- Approved via Emergency Use Authorization by United States FDA for use in children ages 5-11 on October 29, 2021
- Booster shots FDA approved via Emergency Use Authorization for all adults in the United States on Nov. 19, 2021
- Booster shots FDA approved via Emergency Use Authorization for children ages 12-15 on January 3, 2022.
- Moderna
- Viral Vector Vaccines for COVID-19
- Johnson and Johnson
- Approved by United States FDA via Emergency Use Authorization on February 27, 2021
- Booster shots FDA approved via Emergency Use Authorization for all adults in the United States on Nov. 19, 2021
- Johnson and Johnson
- Protein Subunit Vaccines for COVID-19
- Novavax
- Approved via Emergency Use Authorization by the FDA for use in adults on July 13, 2022.
- Novavax
- Inactivated Virus Vaccines for COVID-19
COVID-19 mRNA Vaccines
The mRNA vaccines under development for COVID-19 include the Moderna and Pfizer/BioNTech vaccines. Both of these rely on genetic material called mRNA (short for messenger RNA) in order to stimulate the body’s cells to generate antibodies which can neutralize SARS-CoV-2, the virus that causes COVID-19 infection.
Nucleic acid vaccines, which refer to vaccines that contain either DNA or RNA, have never been used on the market, so COVID-19 presents a unique “make or break moment” for mRNA vaccines.
Both the Moderna and Pfizer/BioNTech vaccines are comprised of mRNA enclosed in tiny nanoparticles. This mRNA contains instructions for the body to create only the part of the SARS-CoV-2 virus that causes COVID-19 infection — the spike proteins — not the entire virus. The spike proteins are depicted in red in the (by now) iconic photo of the SARS-CoV-2 virus below:

The SARS-CoV-2 spike protein uses its spiky features to get into human cells, where it can instruct our cells to make more copies of itself. This replication is what causes COVID-19 infection. Without the spike protein, the virus cannot get into our cells. So, mRNA vaccines work by giving our bodies the instructions to create the spike proteins. The mRNA is read into our cells to produce the actual spike proteins — but just the spike proteins, not the virus itself.

The idea behind the mRNA COVID-19 vaccine is that it gives our bodies the genetic instructions to produce antibodies to the viral spike protein. This “trains” our bodies to recognize and neutralize the spike proteins. The next time the novel coronavirus enters the body, the immune system will work quickly to neutralize it before it can cause a COVID-19 infection.
To watch a short, 2-minute video about mRNA and how mRNA vaccines work, click here.
Let’s learn more about the two most promising COVID-19 mRNA vaccines below.
Moderna’s mRNA Vaccine for COVID-19
Moderna Therapeutics is a biotechnology company headquartered in Cambridge, MA, USA. Their vaccine (mRNA-1273) has the shortest development time of all the vaccines in Operation Warp Speed, with a mere 42 days from mRNA sequence selection to clinical trials. Moderna’s vaccine was also the first vaccine to begin clinical trials in the United States, and on December 18, 2020, this vaccine was the second to obtain clinical approval in the United States via FDA Emergency Use Authorization.

Find out how the Moderna mRNA vaccine for COVID-19 works in more detail over on our blog.
Pfizer/BioNTech mRNA Vaccine for COVID-19
The Pfizer/BioNTech vaccine was developed independently of Operation Warp Speed, the large-scale U.S. government-sponsored initiative to get a COVID-19 vaccine on the market. However, this vaccine (called BNT1262b2) was the first to enter the commercial market, obtaining FDA approval via Emergency Use Authorization on December 11, 2020. The Pfizer CEO, Albert Bourla, was committed to make the vaccine widely available by the end of 2020.
Like the Moderna vaccine, the Pfizer/BioNTech is an mRNA vaccine. Learn more about how the Pfizer/BioNTech mRNA vaccine works.
Viral Vector Vaccines for COVID-19
Viral vector vaccines are a “mashup” of the SARS-CoV-2 virus as well as another virus. The second virus acts as a delivery vehicle for the virus into the body. The two most promising viral vector vaccines at this time are developed by Oxford/AstraZeneca and Johnson & Johnson.
Oxford/AstraZeneca Viral Vector Vaccine
The technology for this vaccine was initially developed by the Jenner Lab at Oxford University and was not specifically designed for COVID-19. However, shortly after the pandemic, the Oxford researchers shifted priorities to quickly develop a COVID-19 vaccine. In this process, they also welcomed biotech giant AstraZeneca to the research program to assist with clinical testing and production.
This vaccine is a viral vector called ChAdOx1 nCov-19. It gets this name as a “mashup” of a small segment of the SARS-CoV-2 virus (nCov-19) and a chimpanzee adenovirus (ChAdOx1). The viral vector has been cut-and-pasted together in the laboratory. It is not capable of making more copies of itself; its only purpose is to deliver the SARS-CoV-2 genetic material into our bodies so that our cells can produce the viral spike protein. This way, our bodies develop immunity to the spike protein so that, when another SARS-CoV-2 virus comes around, we will be able to rapidly neutralize the virus by identifying and breaking down the spike proteins. This is the mechanism by which the Oxford/AstraZeneca vaccine prevents the virus from entering our cells to make copies of itself and cause a COVID-19 infection.
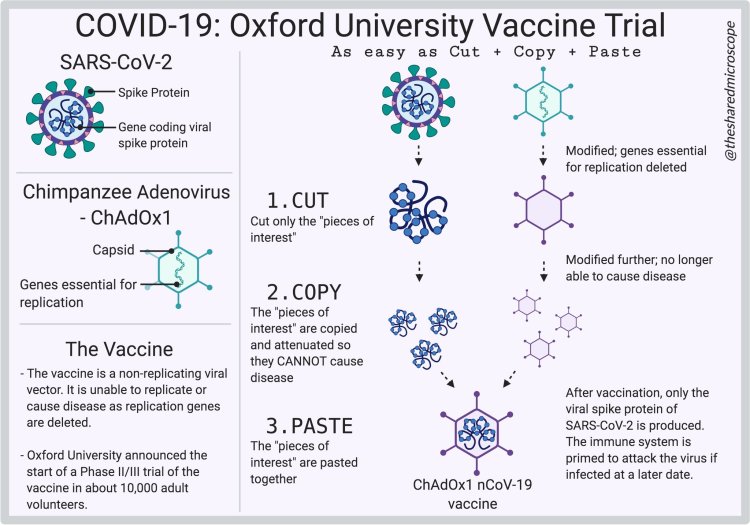
Learn about how the Oxford/AstraZeneca vaccine works.
Johnson and Johnson COVID-19 Vaccine
The Johnson and Johnson vaccine is another viral vector vaccine currently under development. Produced by Janssen, J&J’s pharmaceutical subsidiary, the vaccine is, like the Oxford/AstraZeneca vaccine, based on adenovirus vector technology. Specifically, the J&J vaccine uses a human adenovirus vector to deliver the genetic information for a specific form of the SARS-CoV-2 spike protein into the body. The novel coronavirus genes are delivered to the body via the vaccine to establish immunity to COVID-19.
Learn about how the Johnson & Johnson vaccine works.
Protein Subunit Vaccines
Protein subunit vaccines work by establishing immunity to a particular part of a protein called a subunit.
Novavax COVID-19 Vaccine
The Novavax NVX-CoV2373 vaccine for COVID-19 is made up of laboratory-grown SARS-CoV-2 spike proteins.

Learn about how the Novavax COVID-19 vaccine works and how it is manufactured.
Inactivated Virus Vaccines
Inactivated virus vaccines are made by using heat or chemicals to kill the virus. Because inactivated vaccines do not contain live virus, they cannot cause disease. Inactivated vaccines date back to the 19th century, when researchers observed that killed bacteria could evoke a protective immune response. Inactivated vaccines were subsequently developed to prevent typhoid, cholera, and other bacterial conditions. In 1936, the first inactivated viral vaccine was developed against influenza.

Sinovac’s CoronaVac Inactivated Virus Vaccine for COVID-19
While not part of Operation Warp Speed, Chinese biotech company Sinovac has developed the most successful inactivated virus vaccine currently in clinical trials. The vaccine, called CoronaVac, contains a killed version of the SARS-CoV-2 virus. When introduced into the body, this inactivated virus can facilitate antibody production without causing disease.
Learn about how CoronaVac works.
This site is part of a larger COVID-19 resource page, a collaboration between Fancy Comma, LLC and The Shared Microscope, which can be found here.